This essay is written by Jongsik Jon Chun, a professor at the Department of Biological Sciences, Seoul National University and translated by ChunLab staffs. The author is also actively engaged in R&D as a major board member at ‘ChunLab, Inc.
The possibility of curing Alzheimer’s demonstrated via mice.
Alice: I wish I had cancer.
John: Don’t say that.
Alice: No, I do. I mean it. I mean I wouldn’t feel so ashamed people have cancer they wear pink ribbons for you and go on long walks and raise money and you don’t have to feel like some kind of a social… I can’t remember the word.
In the movie “Still Alice,” Alice who is a professor at Columbia University is diagnosed with one of the most common forms of Dementia, Alzheimer’s disease at the age of 50, during a time one should enjoy life to its fullest. She gradually becomes forgetful and loses memory. Although Alzheimer’s does not involve severe pain as other incurable diseases such as cancer, it is a terrible disease where one gradually experiences the memory loss, and finally one’s spirit. It puts her into an unbearable situation that made her to say that she would rather suffer from cancer.
According to Alzheimer’s Disease International (ADI), the number of worldwide cases of diagnosed with Alzheimer’s 2015 was 46,800,000, but it is forecasted the cases will surge to 135,000,000 by 2050. Previously, infectious disease was the biggest threat in humans, but with the rise of antibiotics which cure many of those pathogenic diseases, cancer has now become the leading issue in our time. Would this be the case in the future as well? With the advent of innovative anticancer drugs including personalized anticancer immunotherapy, cancer cases will also cease to decline. On the other hand, cases of Alzheimer’s will soar, not only in Korea but also globally. Currently we have a lack of appropriate treatments. Additionally, the news of global pharmaceutical giants such as Biogen, Eisai, Eli Lilly, AbbVie failing their clinical trials only doom our future of conquering the disease.
However, there is a field that offers a totally different perspective with regard to the existing treatment which is “microbiomes” It is based on the “Gut-brain-axis” hypothesis which suggests the gut and the brain is connected and by controlling the ecosystem of intestinal microbiomes, we can cure brain disease. Recently, a Korean medical team’s groundbreaking report was published in a renowned medical journal named “The Gut Journal.” I would like to briefly share the findings with the readers as it captures the possibility of effective Alzheimer’s treatment.
The biggest difficulty in conducting medical studies related to brain disease is probably the securing of the right laboratory animal. With research ethics in mind, researchers cannot conduct experiments on human before they collect substantial experimental evidence. So, researchers use laboratory animals, especially mice for early stage studies. The laboratory animal has to display symptoms similar to that of the human in order for researchers to effectively explore the disease. For instance, mice and humans show similar symptoms when they develop skin cancer. In the contrary, if we consider depression as an example, how would we diagnose depression in a mouse? Even I as a human being often get confused sometimes trying to figure out whether I am depressed or not! I cannot imagine how difficult it would be to accurately diagnose a mouse with depression. This is the primary obstacle in studying Alzheimer’s. Hence, finding an appropriate laboratory animal model capable of developing into an Alzheimer’s-like disease is critical to conduct full-fledged research.
Professor Inhee Mook’s team at Seoul National University School of Medicine has successfully developed a mouse that naturally carries a disease that is highly similar to Alzheimer’s of humans1. They published their paper in an international journal under the name ADLP(APT) in 2018(https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-017-0234-4). From now on the name of the mouse born via this genetic manipulation will be called “AD mouse” which is a short form of Alzheimer’s disease. The AD mouse clearly manifests various symptoms of Alzheimer’s. First, two lumps of protein, beta-amyloid and tau, are found in the brain of the mouse. As the disease develops, the lump sizes increase and the mouse’s brain cells die out. Moreover, like Alice from the movie, the mouse experiences memory loss degrades its cognitive ability. As Professor Mook’s team secured a suitable laboratory animal, it can be said that they built a strong foundation in the global-scale dementia research through this “AD mouse”. This is what we call “basic” research in the field of biotechnology. The basic research needed in order to develop anti-dementia drugs is to secure the appropriate laboratory animal for testing dementia.
Professor Mook who is an expert in dementia conducted a joint research with Professor Bae’s (Professor Bae is a renowned microbial ecologist in Korea) team at Kyunghee University. To the readers who did not major in biology, the news itself might not be a huge astonishment. However, to me, this is the birth of a prestigious team, the convergence of the two totally irrelevant fields which create incredible synergy.
The correlation between dementia and microbiomes has been revealed by multiple studies already. More than half of dementia patients have chronic intestinal disease and the intestinal microorganism compositions from dementia patients differ substantially from that of the normal people. Then which comes first? Do microbiomes change when dementia develops or does dementia develop because of microbiomes?
The AD mouse Professor Mook’s team developed displays the initial symptoms of dementia at two months since its birth. The two months old AD mouse is the representation of adolescence stage in human. It was shown that the AD mouse at this two months stage already possesses a considerably different microbiome when compared to that of the normal mouse. The hypothesis the researchers had was if microbiomes are the cause of dementia, the disease will progress slowly when the AD mouse’s microbiome is replaced by a normal mouse’s microbiome. To verify this hypothesis, the team performed an experiment of gathering the microorganisms from a normal mouse’s stool and transplanting it into a two months old AD mouse. They conducted this experiment for an intense four months with every daily interval!
To be exact, the paper said that the team performed this experiment “nearly every day” which made me smile. (Yes, I do understand that graduate students and researchers also need some days off too.) The reason why they had such intense research was probably to transplant the normal microbiomes into the AD mouse’s intestine with unerring precision. If it were me, I would have transplanted once in every two days. With all my respect, I take my hat off to the team.
Four months in, the team has three types of mice: a six months old normal mouse which is equivalent of age 30 in human, an AD mouse, and a transplanted AD mouse. The normal mouse is still young so it shouldn’t show any symptoms of dementia but the AD mouse should exhibit various symptoms due to gene manipulation. Then, what do you think should happen to the AD mouse which had the intestinal microbiomes transplantation from a normal mouse?
Firstly, let us take a look at the composition of intestinal microbiomes. The three groups had microbiomes composed with different bacteria. In the case of human, dementia patients show low species diversity but in the case of the mouse, the AD mouse actually displayed higher species diversity. The importance here is that the normal mouse and the AD mouse had considerably different microbiome composition. Additionally, the transplanted mouse had another different microbiome when compared to those of the former two. This is probably because it is a hybrid of those two.
Secondly, the team looked at the degree of accumulated beta-amyloid which serves as a typical yardstick for dementia patients. The six months old normal mouse obviously did not show any beta-amyloid as expected. However, the transplanted mouse surprisingly displayed a significantly less beta-amyloid when compared to that of the AD mouse. (Please refer to the micrograph below) These two mice were the same type of mice until two months of age, later on. One of the mouse’s microbiome was altered.
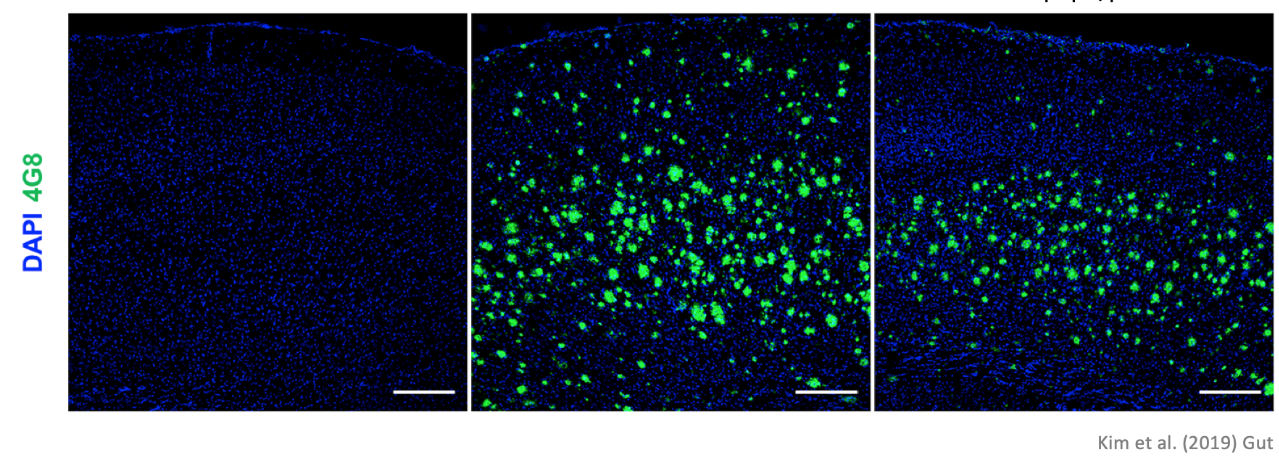
The substance dyed in green is beta-amyloid protein typical of dementia. We can see that the transplanted mouse’s brain has significantly less of the matter compared to the AD mouse
Similarly, the number of tau protein lumps in the transplanted mouse was less than 50 percent than that of the AD mouse, which shows a considerable decrease. (Please refer to the micrograph below) The experiment showed that the microbiome transplantation resulted in the substantial decrease of accumulated typical lumps of two proteins in dementia patients
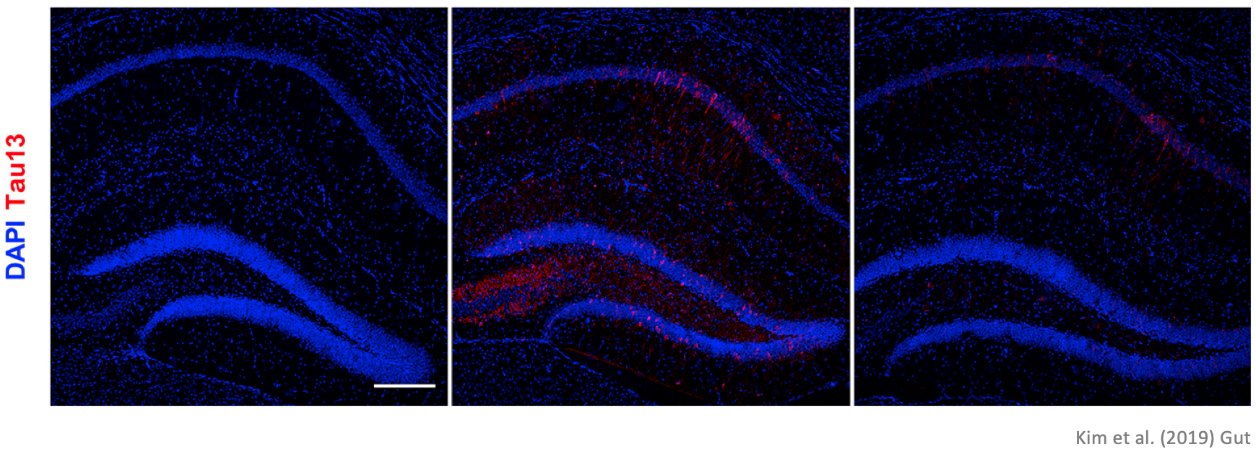
The substance dyed in red is tau protein. We can see that the transplanted mouse’s brain has significantly less of the matter compared to the AD mouse.
Thirdly, the team observed inflammations that occur in the brain also known as neuroinflammation. Inflammations occur when our immune system is weak, and when our body is abnormally activated. It occurs in organs such as intestine or liver, and it can also manifest itself as a systematic inflammation all over the body. There are numerous publications showing that the brain possesses microglia which acts as immunocyte that can lead to inflammations. Studies also demonstrate that these neuroinflammation has a correlation with brain diseases including autism spectrum disorder, Parkinson’s disease, and dementia. Then with the microbiomes transplanted into the body, would there be a decrease in neuroinflammation?

Brain cells with neuroinflammation have been dyed in red and green. We can see that the transplanted mouse’s brain has comparatively less inflammations than the AD mouse.
Based on the results above, the level of inflammation has certainly dropped. This shows that by altering microbiomes, the neuroinflammations which worsens brain diseases can be decreased.
The movie “Still Alice” portrays the painful process of Alice losing memory. Then lastly, how has the mouse’s memory been impacted by microbiome transplantation? The research team used the Y-maze to test the short term memory.
The Y-maze test measures short term memory by calculating the probability of making the correct choice based on short-term memories. According to the test results, the transplanted mouse had memory not as good as the normal mouse but much better than the AD mouse. This leads to the conclusion that microbiome transplantation not only improved various biological indexes related to dementia but also improved cognitive ability. Furthermore, the result presents the possibility of treating Alzheimer’s disease using microbiomes.
Of course, this experiment has been conducted on mice and we cannot guarantee the same results to be shown in humans. However, it suggests another means of developing new treatments. Moreover, the microorganisms from the stool used in transplantation can differ from donor to donor. Therefore, the experiment also highlights the need of microbiome agents in developing into a specified drug. I believe follow-up studies related to the development of new drugs and diagnosis will be conducted in various fronts. As a matter of fact, researchers from Wisconsin University have already started clinical research transplanting the stool microbiomes to Alzheimer patients.
Through the study outlined above, we have found the possibility of stopping Alzheimer’s disease by controlling intestinal microbiomes. If intestinal microbiomes are truly important in this disease, wouldn’t there be a possibility to cure dementia with intestinal microbiomes? In 2015, the total cost of managing dementia in South Korea recorded 13 trillion Korean Won, and is estimated to surpass a 106 trillion Korean Won by 2050. As someone who is affiliated with this field, I sincerely hope the inventions of new drugs using microbiomes could lead to a successful prevention and treatment of Alzheimer’s before the massive-budget-consuming calamity comes. Active follow-up studies and investments should be involved, and I give my word that I will fully support and contribute to this critical issue of our time.
Reference
- 1.Kim M-S, Kim Y, Choi H, et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut. Published online August 30, 2019:283-294. doi:10.1136/gutjnl-2018-317431

