General Characteristics
Streptococcus mutans is a gram-positive cocci bacteria that can grow under both aerobic and anaerobic conditions. It is commonly found in the human mouths and is known to be highly associated with tooth decay which greatly affects an individual’s health. S. mutans was firstly described as caries causing strain by J. Kilian Clarke in 19241. The bacteria is also a representative “cavity-causing strain” of Streptococcus group along with the strain, S. sobrinus2. In 2002, the genome sequence of the S. mutans UA159 revealed the genomic size of 2,030,936 bp and GC ratio as 36.82%. It has 1,963 ORFs, metabolizes various carbohydrates through non-oxygen pathways and about 15% of the genome is consisted of genes related to the transport system1.
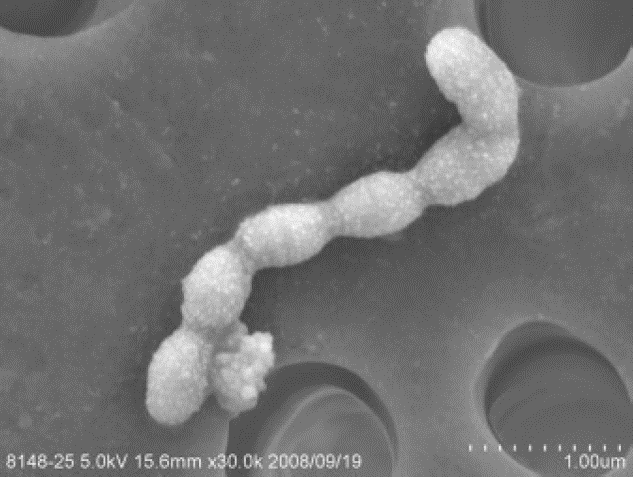
Clinical importance: Tooth decay
Streptococcus mutans has various virulence factors that contribute to tooth decay. Firstly, it produces lactic acid through metabolism. Secondly, it produces water-insoluble glucans which help bacteria to bind to the surface of tooth. Finally, the most important virulence factor of the S. mutans is the acidophilicity which makes S. mutans to thrive under acidic conditions. Under the reduced pH environment caused by lactic acid production, S. mutans can perform higher metabolism than the other bacteria in the oral cavity. S. mutans become the dominant pathogen in the oral microbiome, and the decreased pH causes the tooth surface to melt (demineralization) and leads to the formation of cavities3,4.
Like any other infectious pathogens, Streptococcus mutans rely on transmission pathways to propagate amongst human hosts. It is known that S. mutans are not found in infants until the eruption of their primary teeth because S. mutans require a firm, non-shedding surface as of the tooth to settle. Generally, this bacteria can settle in the oral cavity at the age of one and two and causes tooth decay, which is known as Early Childhood Caries(ECC). The most common route of transmission of S. mutans is a vertical infection which is transmitted directly from mother to child. The early evidence of the vertical infection was confirmed through bacteriocin typing study, chromosomal DNA pattern,and plasmid similarity comparison, etc. According to the study conducted by Berkowitz’s research group5, when the mother had more than 10⁵ colony forming units (cfu) of mutans streptococci(MS) per mL of saliva, the probability of transmission to infants was 58%, but at 10³ cfu, it showed 6% with about 9 times decreases. This illustrates that the density of S. mutans in mother’s saliva is a significant risk factor for infants’ early infection3,5. Meanwhile, S. mutans can also be spread through horizontal infections. The same S. mutans strains were presented among children in the same nursery class in many cases6, and children who were not detected with S. mutans until the age of 5 have been observed to be sharing the strain with their parents when they were subsequently infected7. Frequent contact with others appears to be sufficient to propagate S. mutans, which means that in a daycare center-like environment, the likelihood of children becoming infected with each other may increase.
In relation to Microbiome
With the development of NGS technology, the microbiome studies of the human oral cavity have been actively conducted, and S. mutans is being used as an important biomarker in these research studies. Tanner et al.,8 analyzed the oral microbiome of the adolescent populations of Romania and Sweden and they found S. mutans is detected in the oral microbiome of Romanian adolescents who have severe cavities. In another study, it was discovered that the consumption of milk is effective in changing the oral microbiome and reducing cavities9. In the group with low intake of milk, S. mutans were found more frequently than in the group with higher intake of milk. Additionally, the results were the same as the previous study which suggested that the existing milk proteins and peptides show blocking effect of S. mutans adhesion and metabolism. Likewise, many researches are being conducted with various perspectives using oral microbiome analysis to confirm and study the infection and prevention of S. mutans.
- 1.Ajdic D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proceedings of the National Academy of Sciences. Published online October 23, 2002:14434-14439. doi:10.1073/pnas.172501299
- 2.Streptococcus mutans. Science Direct. https://www.sciencedirect.com/topics/medicine-and-dentistry/streptococcus-mutans
- 3.Simon L. The Role of Streptococcus mutans And Oral Ecology in the Formation of Dental Caries. Journal of young investigators. Published October 2007. https://www.jyi.org/2007-december/2017/11/10/the-role-of-streptococcus-mutans-and-oral-ecology-in-the-formation-of-dental-caries
- 4.Banas J A. Virulence properties of Streptococcus Mutans. Front Biosci. Published online 2004:1267. doi:10.2741/1305
- 5.Berkowitz R. Mutans streptococci: acquisition and transmission. Pediatr Dent. 2006;28(2):106-109; discussion 192-8. https://www.ncbi.nlm.nih.gov/pubmed/16708784
- 6.Berkowitz RJ, Turner J, Green P. Maternal salivary levels of Streptococcus mutans and primary oral infection of infants. Archives of Oral Biology. Published online 1981:147-149. doi:10.1016/0003-9969(81)90086-8
- 7.van Loveren C, Buijs JF, ten Cate JM. Similarity of Bacteriocin Activity Profiles of Mutans Streptococci within the Family When the Children Acquire the Strains After the age of 5. Caries Res. Published online 2000:481-485. doi:10.1159/000016627
- 8.Johansson I, Witkowska E, Kaveh B, Lif Holgerson P, Tanner ACR. The Microbiome in Populations with a Low and High Prevalence of Caries. J Dent Res. Published online October 6, 2015:80-86. doi:10.1177/0022034515609554
- 9.Johansson I, Esberg A, Eriksson L, Haworth S, Lif Holgerson P. Self-reported bovine milk intake is associated with oral microbiota composition. Milgrom PM, ed. PLoS ONE. Published online March 21, 2018:e0193504. doi:10.1371/journal.pone.0193504

